It’s not the heat, it’s the humidity. But its not the relative humidity.(*)
As a raw number, relative humidity doesn’t tell you anything. The video above shows how the same amount of water produces different relative humidities depending on air temperature.
For example, early yesterday morning in my backyard it was 80 degrees with relative humidity 79%. Last Tuesday it was 66 degrees with relative humidity 83%.
So didn’t yesterday’s 79% humidity feel better than 83% last Tuesday? No! Yesterday’s 80 degrees held a lot more water.
Dewpoint (the temperature at which the air is so saturated that it rains or produces dew) is the helpful number that tells us that. If you know the temperature and relative humidity you can calculate the dewpoint here.
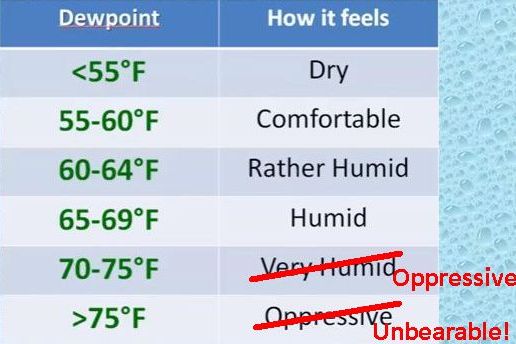
The National Weather Service in Chicago made a chart to describe how we feel at various dewpoints. I’ve marked it in red to show my own heat-averse opinion. (Click on the screenshot to see their dewpoint video that includes this chart.)
So here’s what was really going on this week and why it felt so hot yesterday even though the temperature never reached 90 degrees. Notice that the relative humidity was at its lowest yesterday afternoon.
| Date/Time | Temperature | Relative Humidity | Dewpoint | Comfort Range |
| Tuesday Aug 9, 7am | 66oF |
83% |
61oF | Rather humid, almost comfortable |
| Friday Aug 12, 7am | 80oF |
79% |
72oF |
Oppressive |
| Friday Aug 12 afternoon, 2pm | 88oF |
59% |
72oF |
Oppressive |
Find out the dewpoint before you go outdoors and you’ll know whether you want to brave it!
(*) p.s. See the comments!
(video from Richard Clements on YouTube. screenshot from NWS Chicago video. Click on the screenshot to see the video)
Oooh! I love dewpoints but not the way this week’s have made me feel. Something I talk about in my chemistry classes. We can also explain overnight highs using them. Curious? I’ll break it down without all the chemistry with a couple every day examples.
1) There is a property of all substances called specific heat capacity. This is how much heat energy any substance can hold. The higher the capacity, the greater that substance’s resistance to temperature change. We can qualitatively deduce this value by considering the temperature difference between a concrete or asphalt drive way and a grassy lawn right next to it. Which would you rather walk on mid-afternoon? They’ve both been exposed to exactly the same amount of solar radiation heating them up, showering them with heat energy, since sunrise. But the grass is cooler! This is because of heat capacity. The grass has a greater heat capacity. It collects more energy before rising a full degree in temperature. (This also happens to relate to why turf fields, regardless of the advances in materials will always be warmer and aren’t a great place to play games during the day. Prior to the 2015 Women’s World Cup, Alex Morgan shared a tweet of the on field temperature being over 120 degrees F on a turf field that the women would have to play on.)
Same thing with the beach! Go on a sunny day and you’ll be familiar with the burning hot sand next to the sometimes frigid, but always cooler waters. It is an imperfect example because of all the mixing due to the waves, but things are working the same way! Beach sand and water near the shore are receiving the same amount of heating, but the sand is burning hot while the water is a pleasant relief. The grass has a high water content in the previous example and the sea is about 100% water.
Water has a high heat capacity.
2) So what does this have to do with dew points? If we have a high dew point, your post just explained how that means there is a lot of water in the air. We can see this at work if we compare the daily temperature fluctuations here in Pittsburgh with somewhere more desert like…San Jose for example! We reached about the same high temperature as they did during the heat yesterday.
But compare our overnight lows. We bottomed out at 71 this morning. San Jose is still in the dark and cooling down to a forecast 60 degrees this morning! Why do they get to cool off an additional 10 degrees? Their dew point is 15 degrees lower! Their air has less water in it. And the first two examples demonstrated that water has a very high capacity to hold heat energy.
Two side notes –
a) It’s a darn good thing water has this high heat capacity…it helps humans maintain body temperature. Without it, we’d really freeze our toes off in the extreme cold because it acts as a giant reservoir of energy for our bodies. During the summer we sweat, and as it evaporates our sweat takes excess heat energy from our bodies. Again the high heat capacity of water means it can carry a lot of heat away (assuming it evaporates which becomes more difficult in humid environments.)
b) The high heat capacity is why some people point to water as being the worse greenhouse gas. It does hold more heat energy than other gases (namely the nasties carbon dioxide and methane, but really which ever other gas you choose.) Water is condensable though as the youtube you embedded shows! The others aren’t. here’s a pretty good explanation of the difference. https://www.acs.org/content/acs/en/climatescience/climatesciencenarratives/its-water-vapor-not-the-co2.html
If you made it this far, *high five*! (Now to see if there is a character limit to comments here. Pretty sure this is longer than your blog post. Oooops.)
I LOVE SCIENCE!
Your explanation was much easier to understand than the video. Thank you!
Here’s a map of the dewpoints across the country.
http://weather.unisys.com/surface/sfc_con.php?image=de&inv=0&t=cur
Looks like it is going to be sticky all over the East! This would be a good weekend to be in Acadia, Kate.
Peter, Love your explanation! Here in the desert southwest, we look for dew points to rise above 54* for monsoon rains to begin. In the winter, dew points average in the 20’s – 30’s.
http://www.southwestclimatechange.org/feature-articles/southwest-monsoon
Yeah, it’s been like walking through soup the last few days! I am definitely getting too old for this stuff! ?
Donna, I’d actually included Phoenix as my first example then looked up the weather to include some real data. Your dew points have been in the 60s!!! I was expecting 40s. It makes a much more dramatic comparison. You can hit highs in the 90s and lows in the 50s in those situations. The classic case of how you can freeze to death in a desert.
Now wait a minute. Two issues (One with Kate’s original post and one Peter’s post). And yes I really, really do know what I am talking about (PhD in Meteorology from a small technical school in Cambridge MA and Professor of Chemistry, Chemical Engineering, and Engineering and Public Policy at CMU focused on atmospheric chemistry, air quality and climate).
Issue 1. Relative Humidity does matter. We feel hot because we are having a hard time dissipating heat to maintain our core temperature at 98.6 F. We dissipate heat in two ways. First, air that is cooler than 98.6 F can just plain flow by our bodies (as with a fan or a cool breeze) and it carries heat away. The overall heat capacity of the air does matter to that heat transfer (this is where the ChemE part of my multitude of hats comes in) but the temperature and flow rate of the air are a bigger factor. Second, water (aka sweat) evaporates from our skin. It takes heat to turn liquid water into vapor (the enthalpy of vaporization) and so that cools us down A LOT. The same factors influence how fast sweat can evaporate (wind speed especially) but now assuming that our skin temperature is more related to our body temperature than the outside air, the important thing is how much water vapor the air can hold. That IS related to the RH because at 100% RH the air can’t take up any more water vapor and our sweat just drips off, which does almost nothing to cool us. If it is hot and dry, sweat evaporates super fast, which is why dry heat often does not feel that bad, as long as we keep hydrated so we can keep sweating.
Issue 2. Greenhouse gas efficiency has nothing whatsoever to do with heat capacity. What matters is where and how strongly in the infrared spectrum (i.e. what “color”) the gas absorbs infrared light, which is the electromagnetic manifestation of heat (look up blackbody radiation). Actually, what really matters is where the emit, but this turns out to be identical. Basically what happens is that air circulation moves heat upward in the atmosphere until the air gets high enough so that infrared light from that air can escape into space, which is the ultimate heat sink to balance the ultimate heat source (the Sun).
Water absorbs in very broad regions and it is a very, very important part of the system. CO2 absorbs in somewhat narrower regions, different from water, and so participates as well. The key is that the overall water content of the atmosphere is a CONSEQUENCE of the temperature (this is where that video is right on). Earth is a very large, wet, wick, and the atmosphere over the ocean is almost always at about 80% RH. If it gets hotter the 80% will stay the same but that will represent more water vapor which WILL make the planet hotter, but we can’t shove more water vapor into the atmosphere without making it hotter – the ocean and to a lesser extent trees regulate that. So water vapor represents a feedback, which will make Earth even hotter once we do something to cause warming. However, when we add CO2 there is not such a governor (a good chunk goes into the ocean, making it fizzier, which means more acidic, which is more bad news, but a portion stays in the atmosphere for a very very long time – some for a few 100 years and roughly the same amount for 10’s of THOUSANDS of years) and so the CO2 causes the planet to get warmer.
Another aside – the “window” phenomenon is why methane is such a big deal. Methane absorbs infrared light in parts of the IR spectrum where nothing much else does, and so molecule for molecule it is many times more effective than CO2 at warming. The factor depends a bit on the time period you are interested in, but a factor of 30-50 is about right.
In the grand scheme of things, if we were to double the CO2 in the atmosphere from the pre-industrial level of 280 ppm to 560 ppm and nothing else happened (we are at 400 ppm of CO2 now but with other things like methane and such we are closer to 450 ppm CO2 equivalent), Earth would get about 1 C (1.8 F) hotter. This calculation is, I won’t say easy, but straightforward and extremely well understood. Because of this, Earth will certainly also get wetter (RH will stay about the same, absolute humidity will go up) and this “water vapor feedback” will probably add another 1 C to the warming. The exact amount is not quite as certain but it is also fairly well understood. So Earth will get 2 C (3.6 F) warmer. That is the STARTING point for any conversation about climate change and uncertainty. There is very little that we know about (and very little historical evidence from things like ice ages, etc) that is likely to dampen this response, and there are things that are likely to make it somewhat larger. Thus (and because of a whole confluence of climate models, paleoclimate reconstructions, etc) the estimated range of climate response to a doubling of CO2 (the “climate sensitivity”) is about 2-4.5 C, with a best estimate of about 3 C. It is very, very unlikely to be less than 2 C, because those first 2 C are quite straightforward and all of the evidence is that positive feedbacks on top outweigh negative ones. Also, that science is straightforward enough that those numbers (2-4.5 C per doubling) haven’t really changed much since the Charney report to the National Academies in 1979. That means we have known enough to motivate any reasonable person to action for almost 40 years. And yes the Charney report does say that we need to act “now” (1979) before it is too late…
If 3 C doesn’t seem like a lot, the difference between now and an Ice Age is about 6 C.
Neil, thanks for the info on relative humidity. Since I’m a numbers person (I like digits), I’ve always been confused/annoyed that relative humidity’s raw number decreases when the temperature goes up and the weather feels worse. From a scientific perspective it would have been better to turn these two sentences “But its not the relative humidity. As a raw number, relative humidity doesn’t tell you anything.” into “But as a raw number, relative humidity won’t tell you how bad it feels.” I’m hoping my Poetic License is enough to cover the original rendition.
On the subject of climate change, Thank You for explaining the minimum 2-degree rise and methane’s absorption of IR light … and so much more. Wow! We’re toast. Literally.
Now you all know, in case you wondered, what being married to a Chemical Engineer (Pitt ’74 and ’78) is like. I get this stuff all the time.